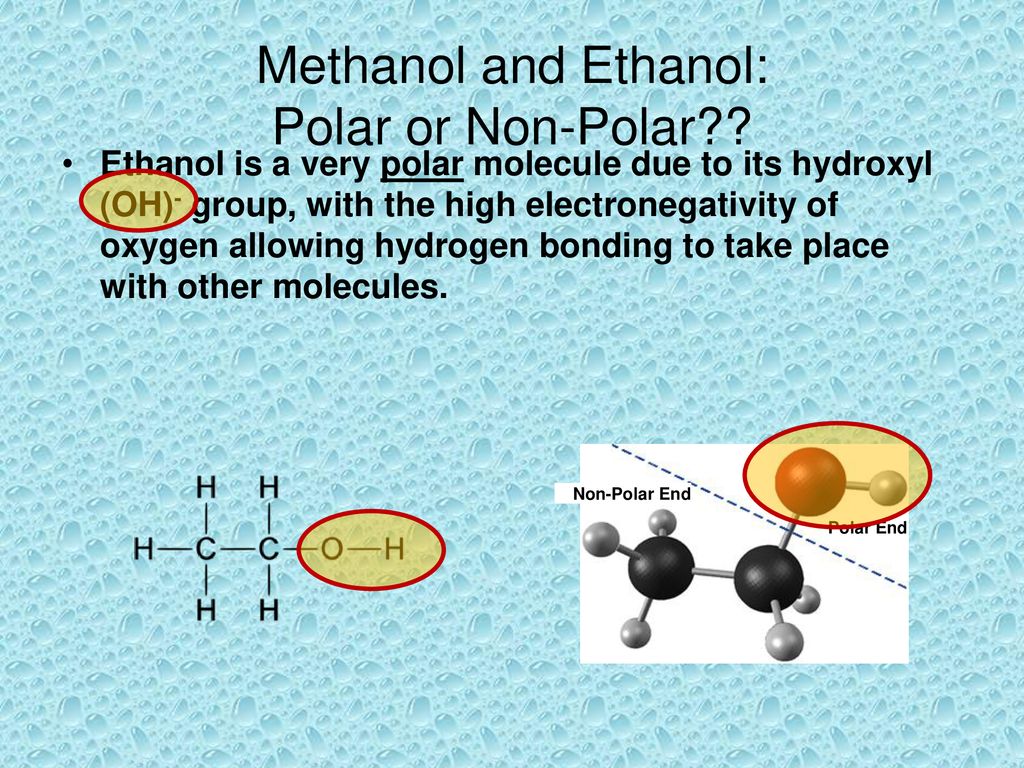
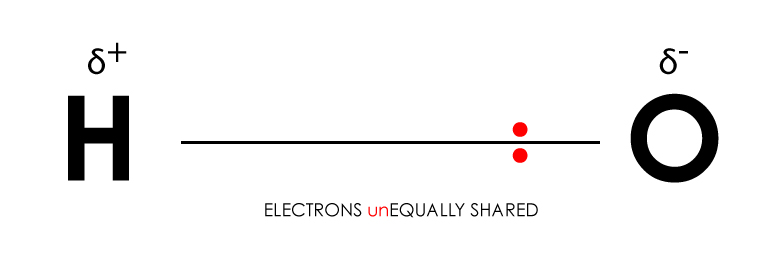
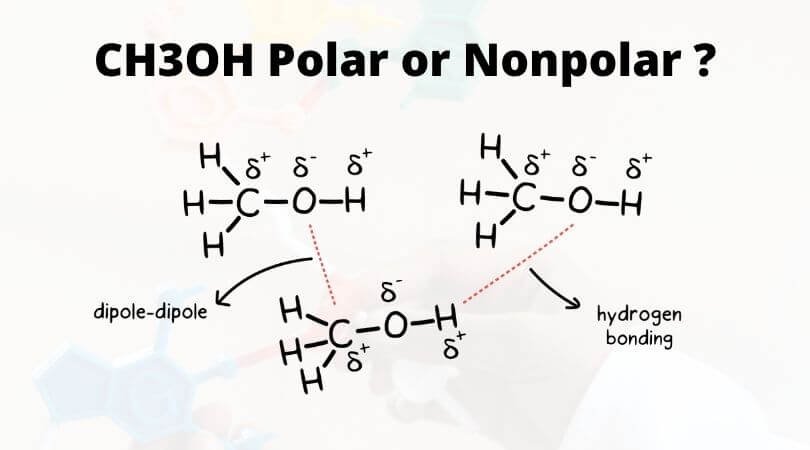
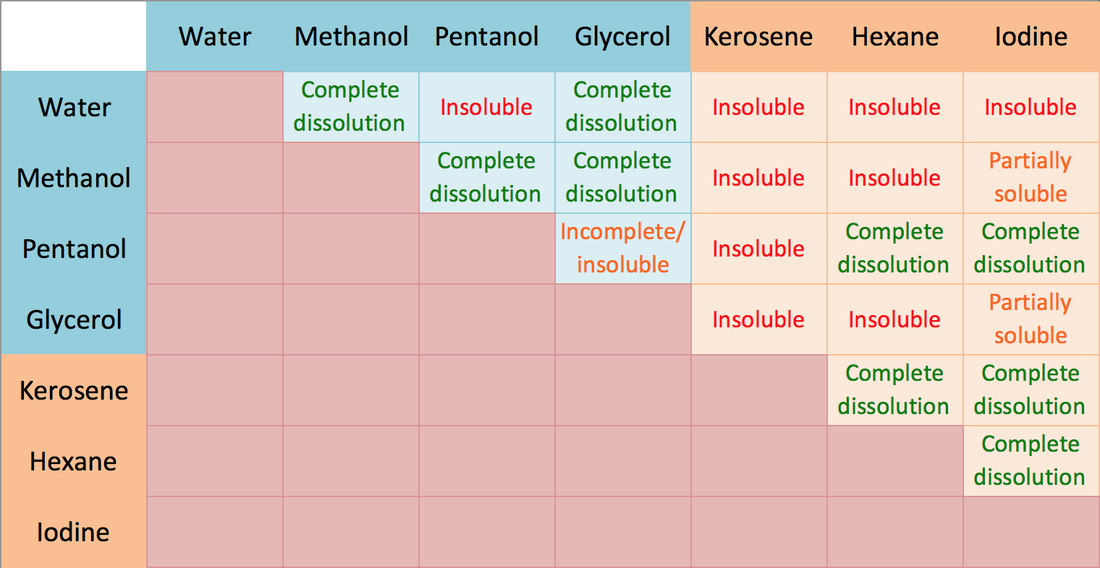
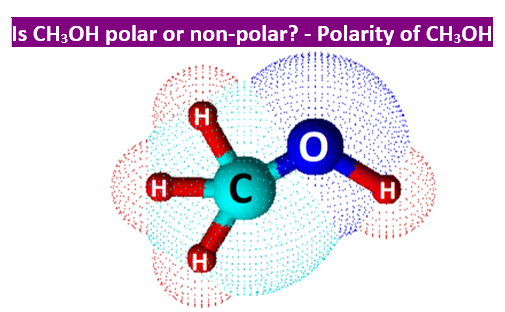
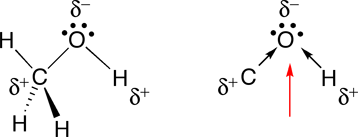SOLVED: Why is methanol (CH3OH) less soluble in hexane (C6H14) than pentanol (I(CH3CH2CH2CH2OH)? Pentanol is a bigger molecule; therefore, hexane can break its bonds more easily. Hexane can form hydrogen bonds with

Vector organic polar aliphatic molecule or solvent of methanol or methyl alcohol CH4O in several variants - organic chemistry concept. Chemical formulas isolated on a white background.:: موقع تصميمي