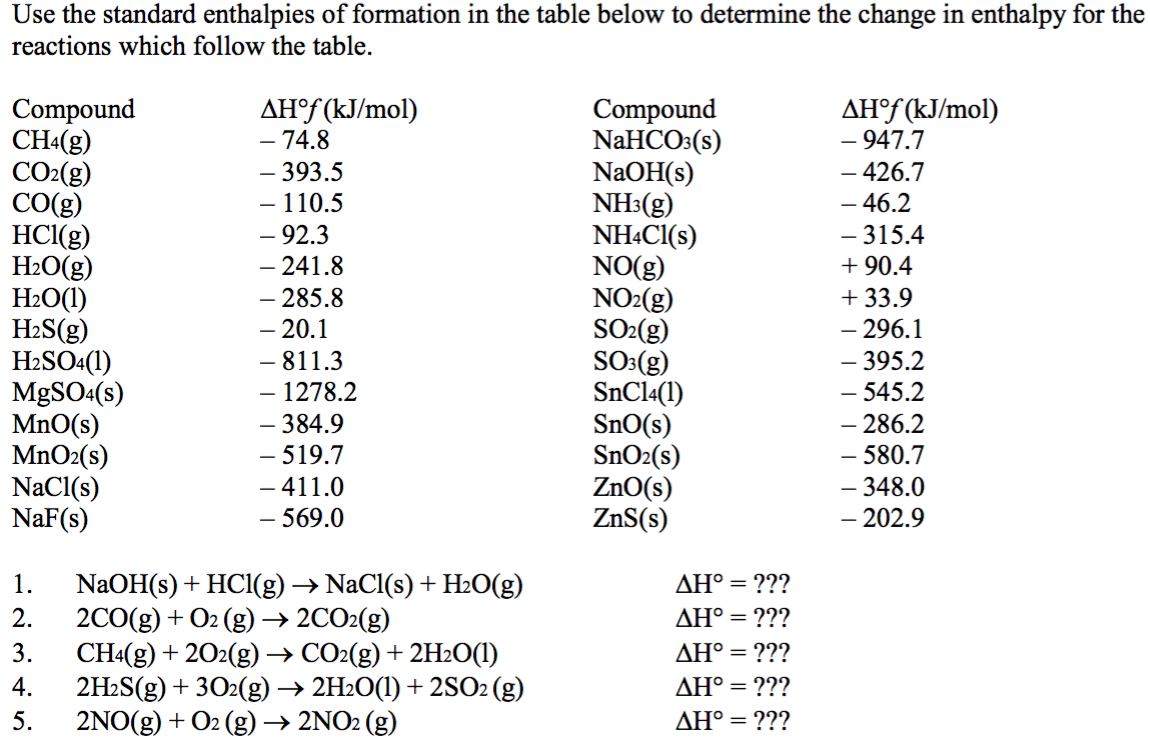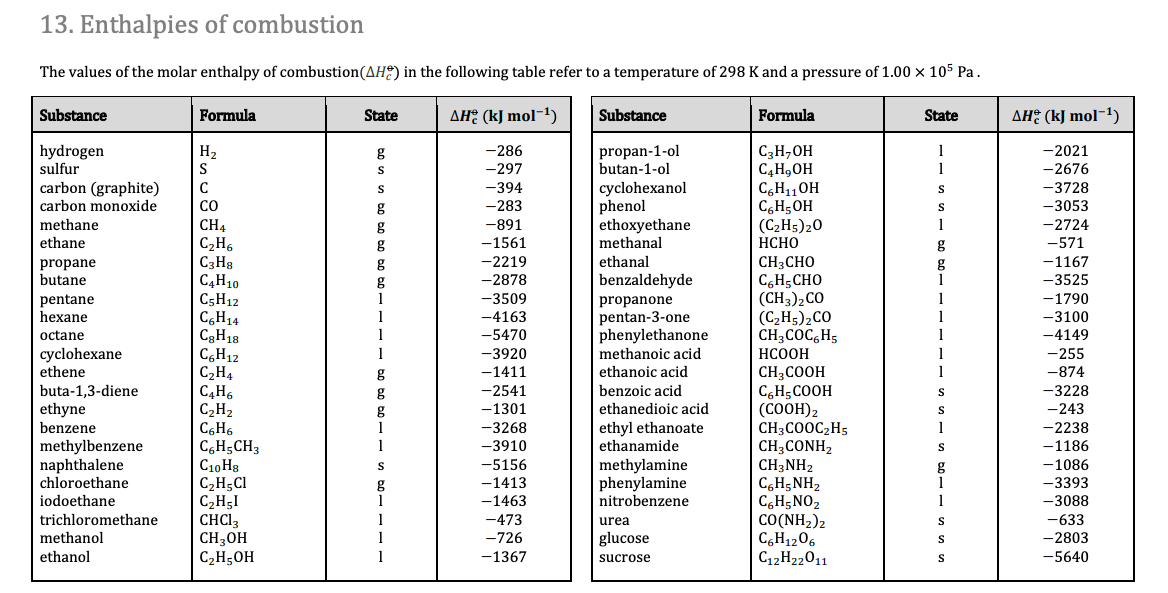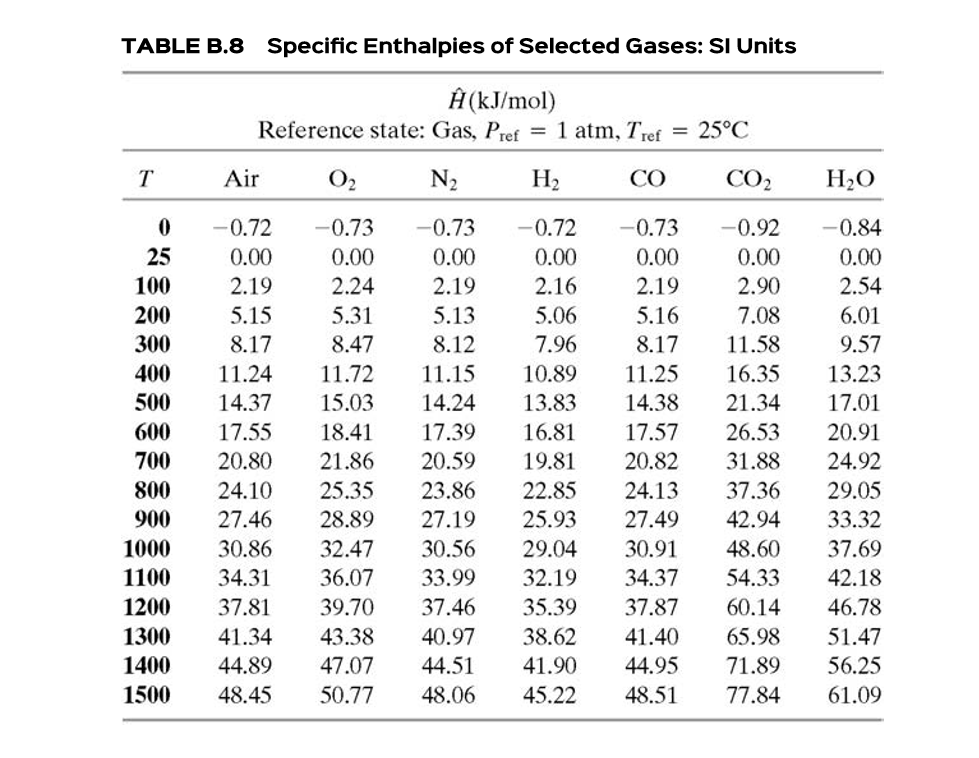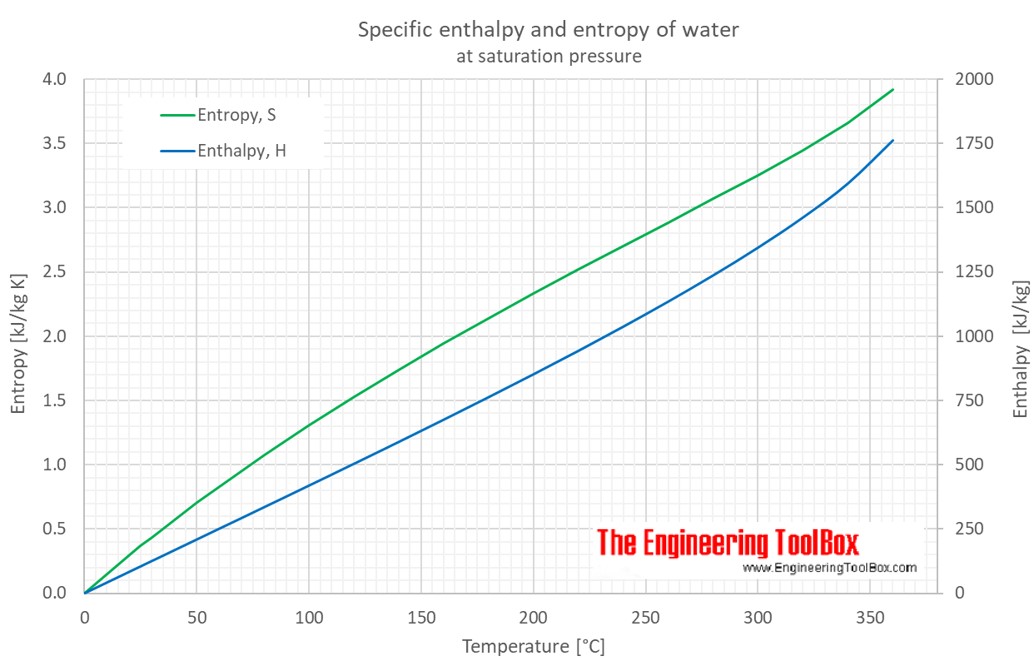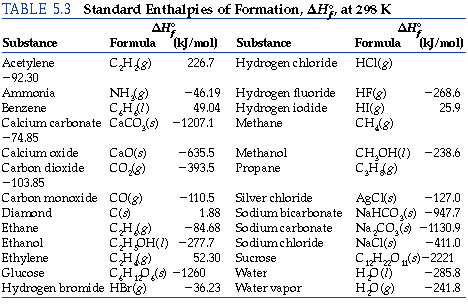
Calculate the enthalpy of reaction for the reaction "CH"_3"COOH" + "H"_2"O" -> "CH"_3"CH"_2"OH" + "O"_2? | Socratic

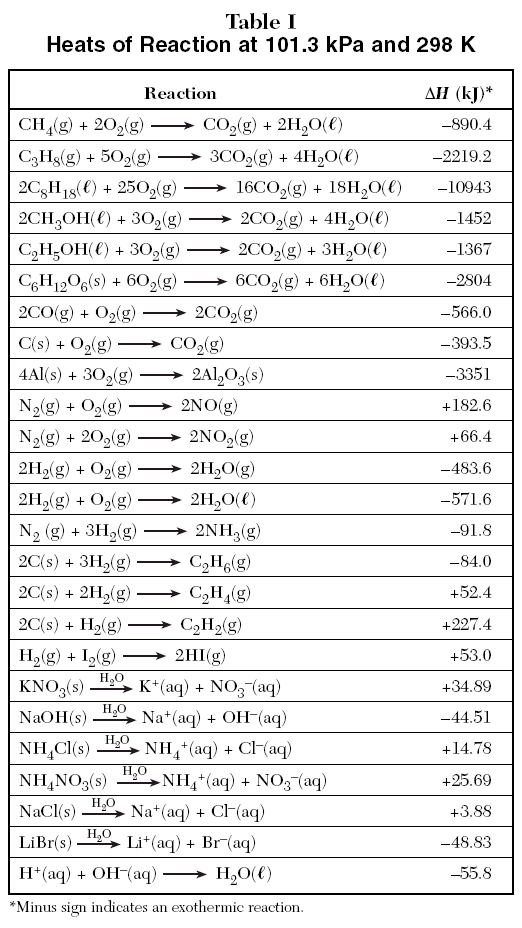
90 POINTSSSS!!! Step 3: Determine the amount of energy change in the reaction. a) Use the table of enthalpy - brainly.com

Table 3 from Group additivity values for enthalpies of formation (298 K), entropies (298 K), and molar heat capacities (300 K < T < 1500 K) of gaseous fluorocarbons | Semantic Scholar
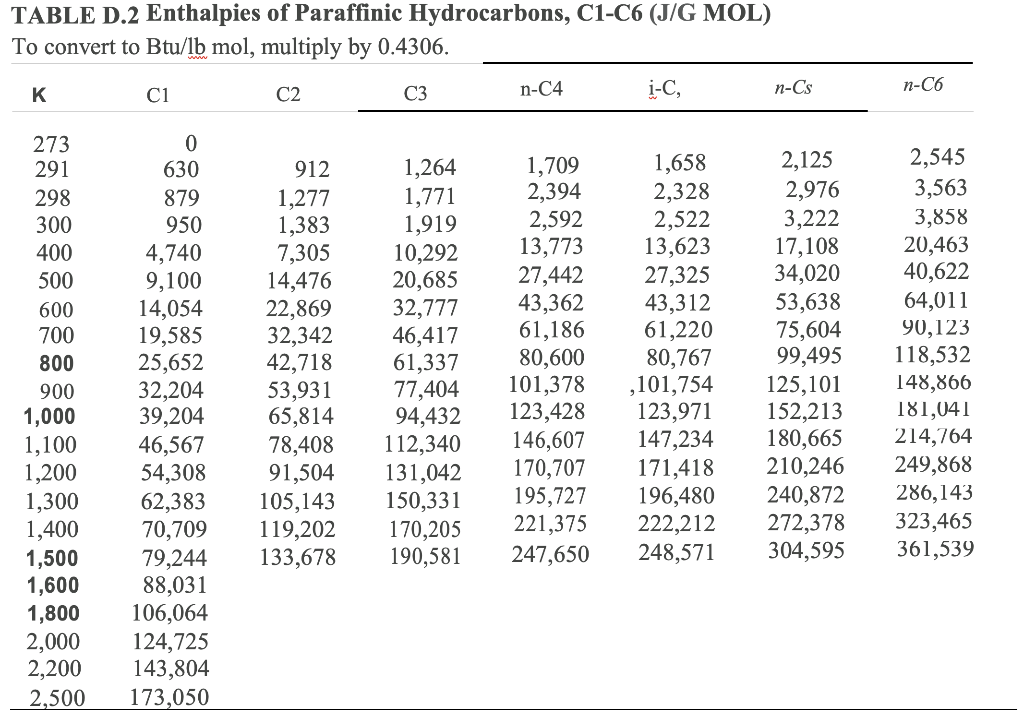
A TABLE OF THERMODYNAMIC PROPERTIES OF HYDROGEN FOR TEMPERATURES FROM 100 TO 3000 K AND PRESSURES FROM 1 TO 50 ATMOSPHERES - Page 96 of 269 - UNT Digital Library

Table 1 from p . 317 – 322 ENTHALPY , ENTROPY AND HELMHOLTZ FREE ENERGY OF TRANSITION AND RARE EARTH LIQUID METALS | Semantic Scholar
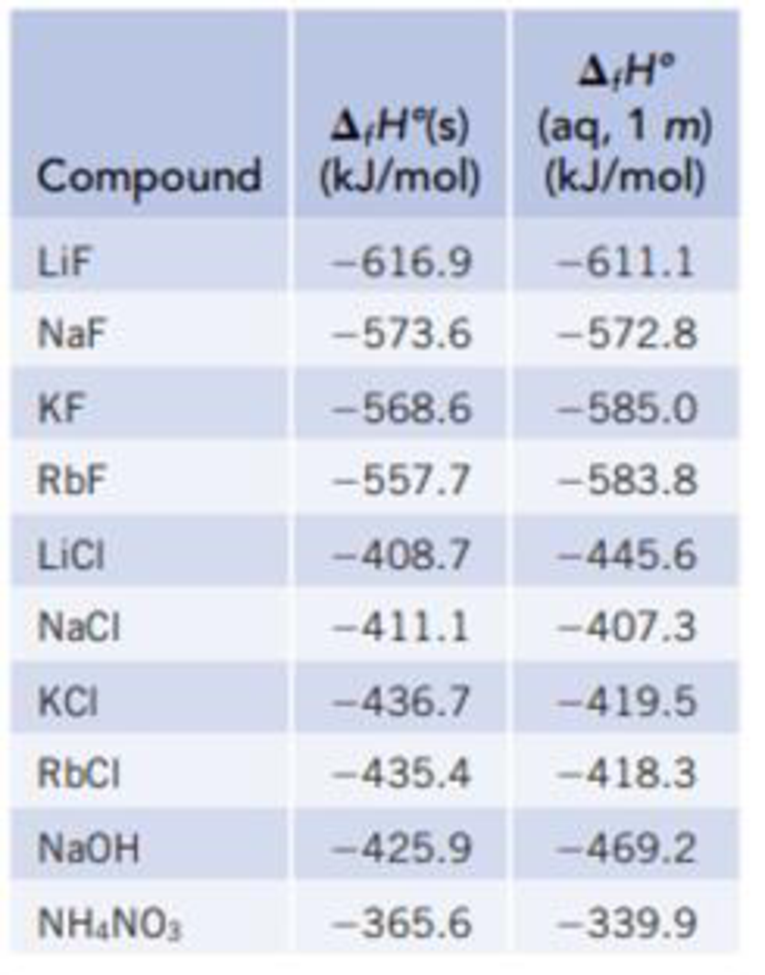
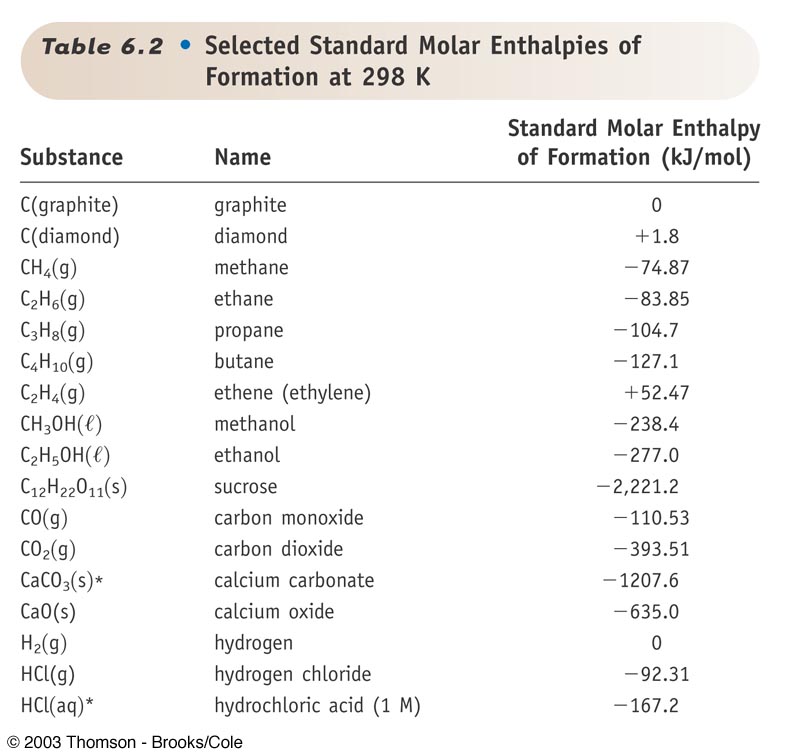
Use the data in Table 13.1 to calculate the enthalpy of solution for NaOH. TABLE 13.1 Data for calculating enthalpy of Solution | bartleby

Table 2 from p . 317 – 322 ENTHALPY , ENTROPY AND HELMHOLTZ FREE ENERGY OF TRANSITION AND RARE EARTH LIQUID METALS | Semantic Scholar
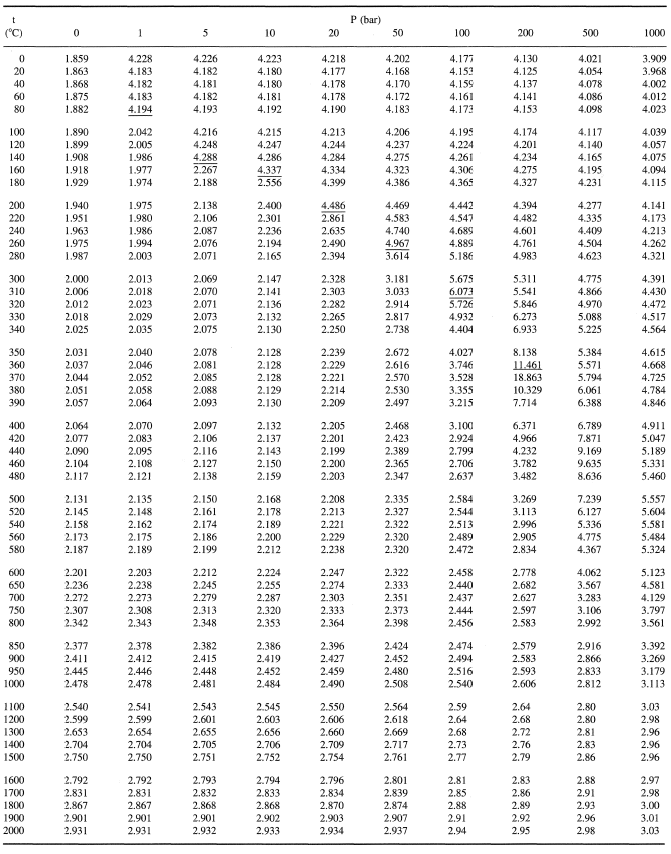
![THERMODYNAMICS] Need help reading a P-h graph. Why doesn't the enthalpy from the graph match the table? : r/HomeworkHelp THERMODYNAMICS] Need help reading a P-h graph. Why doesn't the enthalpy from the graph match the table? : r/HomeworkHelp](https://preview.redd.it/i6twv5gk3i751.png?width=640&crop=smart&auto=webp&s=5235c88aaad6150f01c04ef5996ac26a11b8d0f1)